Targeting the intrinsic ERα disorder
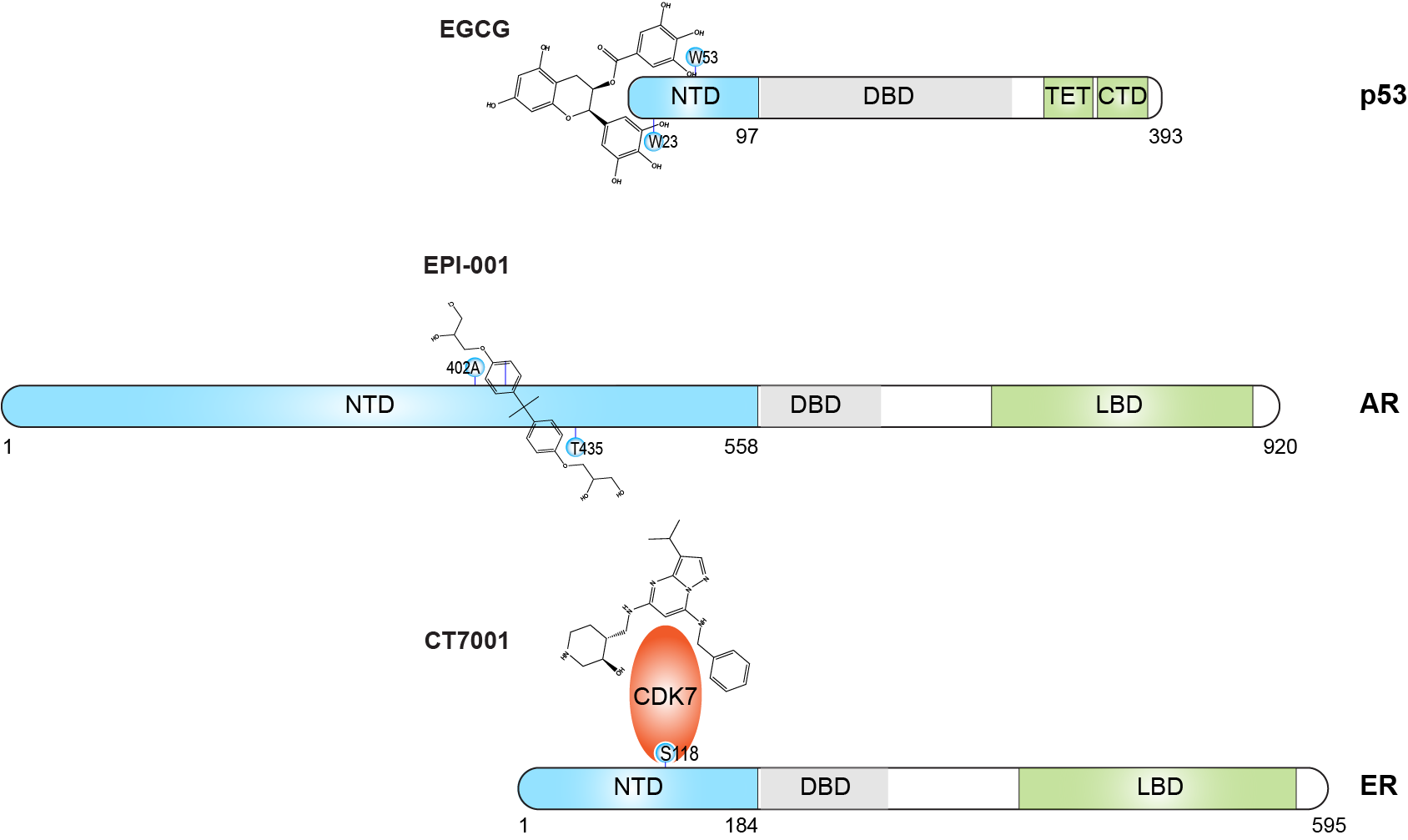
Sex hormone receptors—including estrogen receptor (ER) and androgen receptor (AR)—function as critical transcriptional regulators of cancer cell proliferation. While their intrinsically disordered N-terminal domains (NTDs) have historically presented challenges for drug development, these regions have emerged as promising therapeutic targets due to their essential role in transcriptional regulation.
Unlike the constitutively active AR-NTD (for which inhibitors have been developed over the past decade), the ER-NTD requires phosphorylation at Ser118 for activation. This distinction has led to upstream targeting approaches, including CDK7 inhibitors that block Ser118 phosphorylation. However, these inhibitors present significant toxicity risks due to CDK7's diverse cellular functions.
Our novel approach directly targets the ER-NTD with small molecules, representing a more specific strategy to repress oncogenic ER signaling and inhibit ER-positive breast cancer growth. This approach holds particular promise for addressing challenging clinical scenarios, including tumor-observed ESR1 fusions lacking the hormone-binding domain or male breast cancer that is predominantly ER+ with low estrogen levels.
This work has been funded by the American Cancer Society and Mary Kay Ash Foundation.